The Emergent Microbiome: Rebounding after a Series of Challenging Events
Apr 6th, 2017 by David Puleo | News | Recent News & Articles |
Over the past two years, we have been reviewing and reporting on technology and IP issues related to the emerging field of microbiome research. In this latest piece, we have taken a somewhat different approach, in focusing on the difficult clinical challenges faced by one of the key players in this area and the path forward they have announced to overcome these challenges.
After raising $134 million in its 2015 IPO, Seres Therapeutics, the first microbiome-related biotech to go public, faced a setback last July 2016. Their lead candidate, SER-109, failed to perform significantly better than placebo in reducing recurrent Clostridium difficile infection (CDI) during the ECOSPORTM Phase 2 Clinical Trial (44% CDI recurrence on SER-109 vs. 53% percent CDI recurrence on placebo; more information can be found here on clinicaltrials.gov). As expected, Seres stock dropped, losing 78% of its $1.4 billion market cap. Now, CEO Roger Pomerantz is determined to rebound from the “unexpected” ECOSPORTM data.
Seres is one of the leaders in the microbiome field and has set its sights on treating a number of diseases related to the digestive system, including CDI and inflammatory bowel disease, using its EcobioticTM Platform. This Platform utilizes “combinations of selected microbes that may catalyze a shift of the microbiome from a state that supports disease to a state that supports health.”1 Specifically, the SER-109 lead is an orally administered pill containing bacterial spores from healthy donor patients. Attaining Orphan Drug and Breakthrough Therapy Designations, SER-109 had positive results in an investigator-sponsored Phase 1b clinical trial, prompting progression to Phase 2. After the ECOSPORTM data, Seres analyzed the Phase 2 results and determined that the trial design was somewhat flawed. Pomerantz explains, “We have now identified specific factors that we believe contributed to the Phase 2 results, including issues related to both the accurate diagnosis of C. Difficile recurrent infection, and potential suboptimal dosing of certain subjects in the trial. The SER-109 analyses were recently shared with the FDA, and we are actively discussing the design of a new clinical trial for SER-109.”2 Discussions with the FDA must have gone well because there will in fact be a new trial, where Seres will be able to correct the two above-mentioned shortcomings. Furthermore, Pomerantz and Seres boldly plan to increase manufacturing of SER-109 in anticipation of positive results from the new trial.
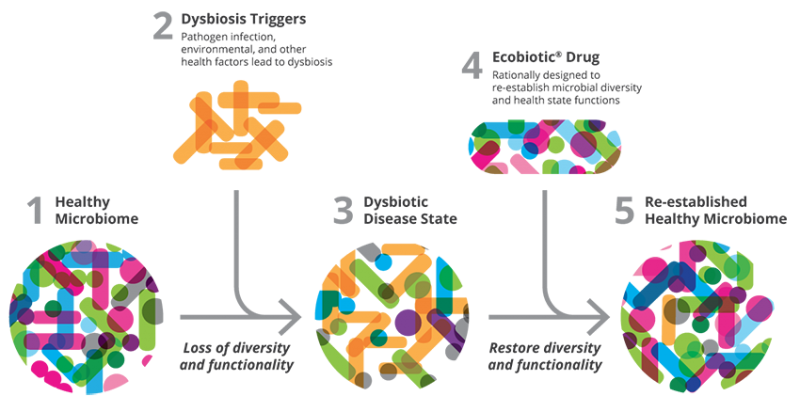
Re-establishing eubiosis using an EcobioticTM drug.1
Seres not only needs to think about SER-109; it also has to think about its backers. In 2016, Seres entered into a collaboration with Nestlé Health Science. As described in Part V of Dilworth IP’s “The Emergent Microbiome: A Revolution for the Life Sciences” blog, Nestlé has a vested interest in the microbiome, specifically using probiotics as “pharmaceutical food products” or nutraceuticals for the treatment of obesity and metabolic disorders. With a stake in the game from Nestlé, Seres is seeking to get its microbiome program back on track.
1 http://www.serestherapeutics.com/our-science/ecobiotic-drugs
2 http://ir.serestherapeutics.com/phoenix.zhtml?c=254006&p=RssLanding_pf&cat=news&id=2240833
-David Puleo and Anthony D. Sabatelli, PhD, JD
David Puleo is a Ph.D. Candidate in the Pharmacology Department at Yale University. He is currently involved in two drug discovery projects targeting tyrosine kinases. Prior to attending Yale, David graduated from Boston College with a B.S. in Biochemistry, after which he worked for two years in the Center for Proteomic Chemistry at Novartis Institutes for BioMedical Research in Cambridge, MA.
This article is for informational purposes, is not intended to constitute legal advice, and may be considered advertising under applicable state laws. The opinions expressed in this article are those of the author only and are not necessarily shared by Dilworth IP, its other attorneys, agents, or staff, or its clients.