Nanomedicine: A Vast Horizon on a Molecular Landscape – Part VII, Quantum dots in medicine
Dec 13th, 2016 by Jing Zhou | News | Recent News & Articles |
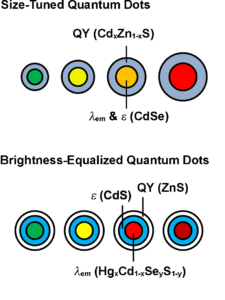
Schematics of the structure of size-tuned Quantum Dots (QDs) and Brightness-Equalized QDs. Drawings used with permission of Professor Andrew M. Smith, Department of Bioengineering, University of Illinois at Urbana-Champaign.
This is the seventh article in a review series on Nanomedicine. We briefly reviewed the major research and entrepreneurial development of nanomedicine and the relevant patent landscape in Part I and Part II of this series. In the next installments we introduced specific applications of nanomedicine, such as Organs-on-a-chip (Part III), nanoparticle drug delivery (Part IV), cancer therapy (Part V), and nanoparticles as biomarkers for imaging (Part VI). Here, we will discuss the therapeutic applications and IP landscape of a special type of nanoparticle known as quantum dots. As in the past, those patent documents cited in the article are summarized in the table at the end.
Quantum dots
According to the Allied Market Research report, the global market for quantum dots will grow from about $300 million to over $5 billion dollars in the period from 2013-2020 period. So, what exactly is a quantum dot and how are they useful?
In 1988, the term “quantum dot” (or “QD” for short) was introduced by Dr. Mark Reed at Yale University to describe nanocrystalline semiconducting fluorophores. Fluorophores are chemical materials that re-emit light when excited by a light pulse. QDs are usually core-shell systems with a semiconductor core enclosed within a shell of another semiconductor material. They usually have confined diameters in the range of 2-20 nanometers (a nanometer is 1 x 10-9 meters) in all three spatial dimensions, resulting in size quantization effects. This size quantization means the band gap (the electron and hole excitation energy levels) of the QD can be “tuned” to provide different light emission frequencies by changing the composition of the QDs and varying their diameters. For example, the larger the QD, the redder, i.e.the lower the energy, emission. Researchers have utilized QDs as efficient materials for advanced photoelectric devices and solar cells. Dr. Arthur Nozik is one of the great leaders in this field (US 4,634,641). During his tenure at the National Renewable Energy Laboratory (NREL), he led a research group to discover variant semiconductor QDs for novel optical and energy systems (US 8,685,781 and US 9,324,562 ). Additionally the surfaces of QDs can be conjugated to various molecules to vary their physical properties, for example, to increase water solubility, reduce cytotoxicity, and resist reactive oxygen formation. The QDs can also be conjugated with specific molecules to target tumor biomarkers. These unique physical properties and the surface chemical modification of QDs have attracted increasing attention to applications in bio-imaging (reviewed in Part VI), bio-analytical assays and diagnostics, as well as the development of new therapeutic agents.
QDs for Cancer Therapeutics
The main therapeutic applications of QDs in targeting cancer include photodynamic therapy, drug delivery, gene silencing, and simultaneous diagnosis/treatment (theragnostics).
Photodynamic therapy (PDT) is a novel clinical management procedure for various cancers, using the combination of light, photosensitizers and molecular oxygen. In general, the photosensitizer absorbs light energy which is transferred to molecular oxygen to generate free radicals as Reactive Oxygen Species (ROS). ROS are potent intracellular cytotoxins, which induce apoptosis of cells (cell death) in contact with the photosensitizer. The current cancer treatment with photosensitizers is not suitable for deeper seated tumors due to the limitation of the light penetration depth, i.e., only a few millimeters. Dr. Clemens Burda’s group at Case Western Reserve University was the first to demonstrate the use of a light energy technique, Fluorescence resonance energy transfer (FRET), to indirectly excite a cadmium-selenium QD conjugated with a silicon phthalocyanine photosensitizer to produce ROS for photodynamic cancer therapy. Dr. John Callan’s group at the University of Ulster, Northern Ireland, demonstrated the use of two-photon excitation to indirectly excite a QD having a cadmium-selenium core and a zinc sulfide outer shell further conjugated to a photosensitizer, to extend the tissue penetration depth of the emitted light. Additionally QDs were conjugated with photosensitizers to improve drug targeting and selectivity. Dr. Dal-Hee Min’s and Dr. Byeong-Su Kim’s groups in Korea developed folic acid (FA)-functionalized carbon nanodots as carriers for zinc phthalocyanine (ZnPc) photosensitizers to spontaneously image and target cancers in photodynamic therapy. Dr. Yury Rakovich’s group at Trinity College in Ireland developed a cadmium/tellerium QD-methylene blue hybrid photosensitizer to target and kill HepG2 and HeLa cancerous cells (WO/2005/105035).
QDs, as nanoparticles, are also potential candidates for drug delivery due to their unique features, such as, large drug loading capacity, enhanced permeability and retention (EPR) effects, long circulation times, targeted drug delivery, and controlled drug release profiles. Furthermore, QDs possess the functionality to trace drug delivery efficiency in real time. Dr. Shuming Nie at Emory University established QD-ABC triblock copolymer systems to target cancer cells and deliver drugs in living animals. These QDs can trace down the drug delivery and cancer treatment process (US 7,846,412 and US 8,394,760). Dr. Andrew Smith at the University of Illinois at Urbana-Champaign (a former member in Dr. Nie’s group, now an independent Principal Investigator (PI) at UIUC), further developed brightness equalized QDs with tunable fluorescence brightness, based on colloidal multi-domain core/shell structures, to precisely match the absorption cross-section and quantum yield (accompanying illustrations, provided with his permission,in this article) (US 2016/0200974 A1). These QDs provide improved performance as fluorescence probes in biological applications. Dr. Yukio Yamaguchi at the University of Tokyo developed a PH sensitive silicon QD drug delivery system, enabling selective intracellular drug release (WO/2012/057253). Dr. Robert Langer’s and Dr. Omid Farokhzad’s groups at MIT developed QD-aptamer-doxorubicin conjugates as a therapy to target prostate cancer cells. The surface of these QDs is functionalized with the A10 RNA aptamer, which recognizes the extracellular domain of the prostate specific membrane antigen (PSMA), a pancreatic cancer biomarker. Once the QD binds to the cancer cell, the conjugated doxorubicin, an antineoplastic anthracycline drug, can kill the targeted cancer cell, while the QD simultaneously images the treatment process in real time (US 2011/0052697 A1).
Besides delivering cancer drugs, QDs can also be utilized to deliver small interfering RNA (siRNA) for gene silencing. In other words, to prevent the expression of certain targeted genes in cancer therapy. Dr. Sangeeta Bhatia’s group at MIT co-transfected siRNA with QDs to track the delivery of nucleic acids via QD fluorescence. Dr. Yong Zhang’s group at National University of Singapore used siRNA delivery from QDs to self-track the gene silencing effects of HER2 siRNA in breast cancer cells (HER2 is usually overexpressed in breast cancer cells). Dr. Ken-Tye Yong’s group at Nanyang Technological University of Singapore used manganese doped zinc selenide QDs for delivering siRNA to pancreatic cancer cells, inducing sequence-specific silencing of oncogenic K-Ras mutations in pancreatic carcinoma. Dr. Xiaohu Gao’s group at University of Washington developed a new technique to combine QDs and amphiopols, which allows efficient delivery and real-time imaging of siRNA in live cells (US 8,063,131).
Summary
As stated above, the market for QDs is expected to balloon to over $5 billion by 2020. In the life science field, QD bioimaging is currently the most mature technique and accounts for most of the market share. The research achievements and economic forces have provided a strong foundation to drive the applications of QD in nanomedicine. Therefore, from the entrepreneurial perspective, many more QD related patents will likely be filed in the coming years.
| Patent Number | Title | Assignee | Inventor |
| US 4,634,641 | Superlattice photoelectrodes for photoelectrochemical cells | The United States of America as represented by the United States | Arthur J. Nozik |
| US 8,685,781 | Secondary treatment of films of colloidal quantum dots for optoelectronics and devices produced thereby
|
Alliance for Sustainable Energy, LLC | Octavi Escala Semonin; Joseph M. Luther; Matthew C. Beard; Hsiang-Yu Chen
|
| US 9,324,562 | Metal halide solid-state surface treatment for nanocrystal materials | Alliance for Sustainable Energy, LLC;
Colorado School of Mines |
Joseph M. Luther; Ryan Crisp; Matthew C. Beard |
| WO/2005/105035 | TARGETED NANOPARTICLES FOR DRUG DELIVERY | THE PROVOST, FELLOWS AND SCHOLARS OF THE COLLEGE OF THE HOLY AND UNDIVIDED TRINITY OF QUEEN ELIZABETH | Iouri K. GOUNKO;
Yury RAKOVICH; Yuri VOLKOV; John DONGEGAN; Dermot KELLEHER; Siobhan MITCHELL |
| US 7,846,412 | Bioconjugated nanostructures, methods of fabrication thereof, and methods of use thereof | Emory University | Shuming Nie; Xiaohu Gao
|
| US 8,394,760 | Multifunctional nanostructures, methods of synthesizing thereof, and methods of use thereof | Emory University | Lily Yang; Shuming Nie; Xiaohu Gao; Xiang Hong Peng
|
| 2016/0200974 A1 | Brightness equalized quantum dots | The Board of Trustees of the University of Illinois | Andrew Smith, Sung Jun Lim |
| WO/2012/057253 | FLUORESCENT SILICON NANOPARTICLES AND METHOD FOR PRODUCING SAME | The University of Tokyo | Susumu INASAWA;
Yukio YAMAGUCHI; Peng SHEN; Seiichi OHTA |
| US 2011/0052697 A1 | Aptamer-Directed Drug Delivery | GWANGJU INSTITUTE OF SCIENCE & TECHNOLOGY;
MASSACHUSETTS INSTITUTE OF TECHNOLOGY; THE BRIGHAM AND WOMEN’S HOSPITAL, INC. |
Omid C. Farokhzad; Sangyong Jon; Vaishali Bagalkot; Liangfang Zhang; Benjamin Teply; Etgar Levy-Nissenbaum; Robert S. Langer
|
| US 8,063,131 | Nanoparticle-amphipol complexes for nucleic acid intracellular delivery and imaging | University of Washington | Xiaohu Gao |
– Jing Zhou, PhD and Anthony D. Sabatelli, PhD, JD
This article is for informational purposes, is not intended to constitute legal advice, and may be considered advertising under applicable state laws. The opinions expressed in this article are those of the author only and are not necessarily shared by Dilworth IP, its other attorneys, agents, or staff, or its clients.

