Rising Carbon Dioxide Levels: Capture Methods & Patent Trends
Jan 22nd, 2019 by Brian Pattengale | Patent Trends & Activity | Recent News & Articles |
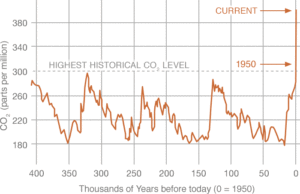 Recent data from NOAA, the National Oceanic and Atmospheric Administration, indicates that current atmospheric carbon dioxide (CO2) levels are at 409 ppm as of October 2018. This is a 36% increase from the highest historical CO2 level,1 and is increasingly being attributed to human activity, namely fossil fuel combustion in power generation, transportation, and industrial processes.
Recent data from NOAA, the National Oceanic and Atmospheric Administration, indicates that current atmospheric carbon dioxide (CO2) levels are at 409 ppm as of October 2018. This is a 36% increase from the highest historical CO2 level,1 and is increasingly being attributed to human activity, namely fossil fuel combustion in power generation, transportation, and industrial processes.
A myriad of issues stem from high CO2 levels, including global warming (1.6 ˚F in the past 35 years), rising sea levels (8 inches in the last century), glacial retreat, and ocean acidification (30% increase in acidity).2 No matter what one’s position is on the debate over the cause-and-effect of human-activity induced climate change, the issue at hand centers around responsible fossil fuel stewardship and controlling CO2 emissions. One strategy is to reduce fossil fuel use by implementing alternative energy sources such as solar, which will be the focus of a future article. This article will serve as an overview of how industrial, academic, and governmental efforts are aiming to tackle the issue of rising CO2 levels, as evidenced by their footprints in the recent patent literature. Particularly, these efforts can be broadly categorized as either CO2 capture methods or CO2 sequestration/conversion methods. The former is the focus of this article, the latter will be the focus of a future article.
While there are multiple methods for incorporating CO2 capture into the fossil-fuel based power generation processes currently utilized, the approach that is most amenable to existing infrastructure is a post-combustion approach where the flue gas exiting a coal or natural-gas fired power plant, consisting primarily of CO2 and water vapor, is subjected to a CO2 capture process. Currently, this is most frequently performed using aqueous solutions of chemical compounds with amine functionalities, such as monoethanolamine, in a CO2 capture unit, also known as a CO2 scrubber. During the operation of a CO2scrubber, post-combustion flue gas is sent through an adsorption column that contains the lean amine solution, where lean indicates low CO2 content. The lean amine undergoes a reversible chemical reaction with CO2, resulting in the formation of rich amines, which are composed primarily of carbamate or bicarbonate.3 Rich amines are then transferred to a desorber system that converts rich amines back to lean amines via competitive water adsorption using steam. This process releases CO2, which can be collected for later conversion steps.
CO2 capture methods have been commercialized and implemented in power plants around the world.5 One prominent example is the Cansolv system from Cansolv Technologies Inc, a sister company of Shell Oil Company. Issued US7056482B2 relates to one of Cansolv’s processes with specific details regarding the mixture of amines utilized, specific properties of the amines, such as the pKa of the amine moeities (a chemical property that is related to proton affinity), and the conditions under which such a system is operable. There are further claims that mention specific oxidation inhibitors to protect the amines from degradation by molecular oxygen in the air, as well as methods to capture SO2 or NOx, other toxic contaminants in flue gas.
In addition to improving these already utilized technologies, other efforts have focused on the development and application of new classes of materials to act as CO2 adsorbers. One such class of materials being developed by an ExxonMobil affiliate (US20180250652A1 & US20180250653A1) are composed of an aluminum oxide support with silicon-modification and an alkali metal salt. The system is reported to improve the overall efficiency of CO2capture by reducing the amount of steam needed during the desorption step. An additional application (US20180250654A1) was filed claiming that the calcination temperature, a heating step in the synthesis of the CO2 sorbent material, has a significant effect on CO2 adsorption ability.
In largely academic environments, a relatively new class of materials is being targeted for CO2 adsorption and separation applications, namely metal-organic frameworks (MOFs). These materials are solid phase and are microporous, meaning that they have typically nanometer-scale pores throughout the material, giving them massive internal surface area and the opportunity to adsorb gasses. Besides casual references to MOFs as a possible sorbent material in the patent literature, scientists are pursuing patents for the application of specific MOF materials to CO2 capture. While many of these examples are in the application stage, they paint a promising future for the emergence of new types of CO2adsorption materials.
All of the previous examples focused on CO2 capture from flue gas, which contains up to 10% CO2, whereas the atmosphere contains only 0.04%.6 From a chemical perspective, it is exceedingly difficult to achieve efficient reaction yield when one of the reactants is at extremely low concentration. Because CO2 capture relies on a reversible chemical process, it follows that performing CO2 capture from atmospheric air with low CO2 concentration is difficult. Despite this challenge, scientists are developing and commercializing processes that are capable of CO2 capture from air. These include Global Thermostat LLC, Carbon Engineering, and Climeworks, among others. Using these technologies to capture carbon from both flue gas and air, it may be possible for power plants to achieve negative CO2emissions, an important first step in remedying the high CO2 levels in our atmosphere.
The table below highlights patents and published applications related to CO2 capture technologies.
| Patent or Application Number | Title | Assignee | Inventor |
| US7056482B2 | Method for recovery of CO2 from gas streams | Cansolv Technologies Inc. | Leo E. Hakka, Michel A. Ouimet |
| US7601315B2 | Process for the recovery of carbon dioxide from a gas stream | Cansolv Technologies Inc. | Michel A. Ouimet |
| US5019361A | Removal of sulfur dioxide from gas streams | Cansolv Technologies Inc | Leo K. Hakka, Paul J. Parisi |
| US10065174B1 | Pelletized immobilized amine sorbent for CO2 capture | US Department of Energy | Walter C. Wifong, McMahan L. Gray, Yee Soong, Brian W. Kail |
| US7314847B1 | Regenerable sorbents for CO2 capture from moderate and high temperature gas streams | US Department of Energy | Ranjani V. Siriwardane |
| Hydrophobic sorbents for CO2/H2O displacement desorption applications | TDS Research Inc., ExxonMobil Research and Engineering Co | Chuansheng Bai, Majosefina Cunningham, Patrick P. McCall, Hans Thomann, Jeannine Elizabeth Elliot, Vinh Nguyen | |
| Application US20180250652A1 | Mixed metal sorbents for CO2/H2O displacement adsorption | TDS Research Inc., ExxonMobil Research and Engineering Co | Chuansheng Bai, Majosefina Cunningham, Patrick P. McCall, Hans Thomann, Jeannine Elizabeth Elliot, Vinh Nguyen |
| Application US20180250654A1 | Calcination of CO2/H2O displacement desorption sorbents | TDS Research Inc., ExxonMobil Research and Engineering Co | Chuansheng Bai, Majosefina Cunningham, Patrick P. McCall, Hans Thomann, Jeannine Elizabeth Elliot, Vinh Nguyen |
| Application US20180117524A1 | CO2 recovery system and method of recovering CO2 | Mitsubishi Heavy Industries Ltd | Tatsuya Tsujiuchi, Takashi Kamijo, Masayuki Inui, Osamu Miyamoto |
| US9855525B2 | Methods and apparatuses for recovering CO2 | Aaron Esser-Kahn | Aaron Esser-Kahn, Du Nguyen |
| US9579602B2 | Catalytic CO2 desorption for ethanolamine based CO2 capture technologies | University of Wyoming | Maohong Fan, Abdulwahab Tuwati, Mohammed Assiri |
| Application US20170216763A1 | CO2 mass transfer enhancement of aqueous amine solvents by particle additives | University of Kentucky Research Foundation | Leland R. Widger, Guojie Qi, Kun Liu, Jonathan Bryant, Cameron A. Lippert, Kunlei Liu |
| Application US20170182453A1 | Metal-organic framework-based sorbents and methods of synthesis thereof | Arizona State University | Bin Mu, Mitchell Armstrong, Allen Wright, Klaus Lackner |
| US9604195B2 | Metal-organic materials (moms) for CO2 adsorption and methods of using moms | King Abdulah University of Science and Technology, University of South Florida | Mohamed Eddaoudi, Michael J. Zaworotko, Patrick Nugent, Stephen Burd, Ryan Luebke, Youssef Belmabkhout, Osama Shekhah |
| US9782745B2 | Metal organic framework, production and use thereof | UTI LP | George Shimizu, Ramanathan Vaidhyanathan, Simon Iremonger, Kyle Deakin, Jian-Bin Lin, Karl W. Dawson |
| US9593132B2 | Metal-organic frameworks (mof) for gas capture | The University of Nottingham | Martin Schroder, Sinhai Yang |
| US9861953B2 | Alkylamine functionalized metal-organic frameworks for composite gas separations | University of California | Jeffrey R. Long, Thomas M. McDonald, Deanna M D’Alessandro |
| Application US20080289495A1 | System and method for removing carbon dioxide from an atmosphere and global thermostat using the same | Peter Eisenberger, Graciela Chichilnisky | Peter Eisenberger, Graciela Chichilnisky |
| US8500855B2 | System and method for carbon dioxide capture and sequestration | Peter Eisenberger | Peter Eisenberger |
| US8119091B2 | Carbon dioxide capture | David Keith, Maryam Mahmoudkhani | Carbon Engineering Ltd. |
| Application WO2018099709A1 | Methods for the removal of CO2 from atmospheric air or other CO2-containing gas in order to achieve CO2emissions reductions or negative CO2emissions | Climeworks Ag | Alexander Spiteri, Valentin Gutknecht, Jan André Wurzbacher, Christoph Gebald |
-Brian Pattengale, PhD and Anthony Sabatelli, PhD, JD
Brian Pattengale is a Postdoctoral Associate in the Energy Sciences Institute at Yale University, where he is investigating the photodynamic properties of emerging materials and their catalytic/photocatalytic applications to reactions such as water splitting or carbon dioxide reduction. Prior to his position at Yale, Brian obtained his Ph.D. in Physical/Materials Chemistry at Marquette University, where he published numerous papers using ultrafast transient absorption and synchrotron X-ray absorption spectroscopies to study functional light absorbing and photocatalytic materials.
1 https://climate.nasa.gov/vital-signs/carbon-dioxide/ – image
2 https://climate.nasa.gov/evidence/
3 https://pubs.acs.org/doi/pdf/10.1021/am507465f – co2 capture review
5 Cebrucean, D.; Cebrucean V. and Ioana Ionel. “CO2 Capture and Storage from Fossil Fuel Power Plants” 2014, 63, 18-26
6 https://www.nytimes.com/2018/02/28/climate/remove-co2-from-air.html
This article is for informational purposes, is not intended to constitute legal advice, and may be considered advertising under applicable state laws. The opinions expressed in this article are those of the author only and are not necessarily shared by Dilworth IP, its other attorneys, agents, or staff, or its clients.

