The A-B-C’s of R-N-A-i: Therapeutic Applications and §101 Implications
Nov 6th, 2018 by David Puleo | Biotech/Pharma | Patent Resources | Recent News & Articles |
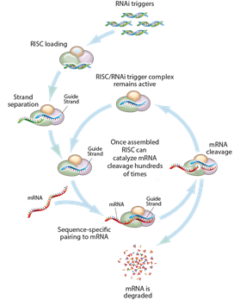 Now that the first RNA interference (RNAi)-based therapeutic, ONPATTROTM (patisiran, developed by Alnylam Pharmaceuticals), recently received FDA approval, it is not surprising that such drugs are gaining considerable attention. However, the underlying technology has been around for quite some time. Craig Mello and Andrew Fire won the 2006 Nobel Prize in Physiology or Medicine for their discovery of RNAi. There has since been a trend towards developing RNAi-based therapeutics, which is reflected in the number of patent applications that have been filed in this area. In this article, we will be reviewing the RNAi methodology and relevant patents, as well as potential challenges for patenting this technology going forward.
Now that the first RNA interference (RNAi)-based therapeutic, ONPATTROTM (patisiran, developed by Alnylam Pharmaceuticals), recently received FDA approval, it is not surprising that such drugs are gaining considerable attention. However, the underlying technology has been around for quite some time. Craig Mello and Andrew Fire won the 2006 Nobel Prize in Physiology or Medicine for their discovery of RNAi. There has since been a trend towards developing RNAi-based therapeutics, which is reflected in the number of patent applications that have been filed in this area. In this article, we will be reviewing the RNAi methodology and relevant patents, as well as potential challenges for patenting this technology going forward.
RNAi is the process of gene silencing or regulation. The theory is that RNAi-based drugs can selectively interfere with RNA content and, thus ,the production of specific proteins in living cells. This selective protein depletion can be used to provide a desired therapeutic effect. For example, if a particular protein is over-expressed in a certain disease state, it is possible to treat the disease by shutting down that over-expression with an RNAi therapeutic (Science 2015 347, 1069).
A large number of patents have already issued relating to RNAi. US7786290 is a general patent regarding RNAi. Although this sounds somewhat straightforward, delivering the RNA has been an essential yet challenging part of the process. Delivery methods include lipid nanoparticles (US20100285112, US7404969, US8642076, US9139554), ligand conjugates (US8106022, US9453222, US9526796), as well as viral delivery. Specifically, conjugation of RNA to N-acetylgalactosamine has proven to be an efficient method (Nature Communications 2018 9, 723).
The first FDA-approved RNAi therapeutic, ONPATTROTM, is used to treat hereditary transthyretin (TTR)-mediated (hATTR) amyloidosis with polyneuropathy. This disease is caused by misfolding of the TTR protein in the liver. Patents US8168775, US9101643, and US9234196 claim this invention. In fact, the FDA Orange Book lists no fewer than 21 patent covering the drug. Alnylam has also partnered with Kumamoto University on targeting TTR that is misfolded in the eye, causing ocular amyloidosis (US20130281510), as well as with Medtronic and the CHDI Foundation for the treatment of Huntington’s Disease (US7320965). Alnylam is targeting other proteins and diseases using RNAi (US7423142, US7605251, US7737265). Other companies in this space include Arbutus Biopharma (US20100285112, US8642076, US9139554), Arrowhead Pharmaceuticals (US9422556), Dicerna Pharmaceuticals (US9200284), and Silence Therapeutics (US7452987).
Given that patent subject matter eligibility is becoming increasingly important in the biotech field, it is likely that questions regarding RNAi will arise. The Federal Circuit recently commented that it expresses “no opinion on subject matter eligibility of method claims that exploit DNA or RNA for drug-like new applications.”[1] Based on the patents presented in this article, it appears that compositions and methods have been deemed patent eligible. However, it is unclear if the court’s stance will change going forward as patents covering RNAi therapeutics will inevitably become the focus of patent litigation and will likely be subject to scrutiny under 35 U.S.C. §101.
| Patent Number | Inventor | Assignee | Title | Issue/Publication Date |
| Disease Treatment | ||||
| US7320965 | Sah et al. | Medtronic Inc | Compositions and methods for inhibiting expression of Huntingtin gene | 2008-01-22 |
| US7423142 | Hans-Peter Vornlocher | Alnylam Pharmaceuticals Inc | Compositions and methods for inhibiting expression of anti-apoptotic genes | 2008-09-09 |
| US7452987 | Giese et al. | Silence Therapeutics AG | Interfering RNA molecules | 2008-11-18 |
| US7605251 | Tan et al. | Alnylam Pharmaceuticals Inc | Compositions and methods for inhibiting expression of the PCSK9 gene | 2009-10-20 |
| US7737265 | Akinc et al. | Alnylam Pharmaceuticals Inc | RNAi modulation of HIF-1 and therapeutic uses thereof | 2010-06-15 |
| US7786290 | Woppmann et al. | Alnylam Pharmaceuticals Inc | Double-stranded ribonucleic acid with increased effectiveness in an organism | 2010-08-31 |
| US8168775 | Wen-Yee Sah et al. | Alnylam Pharmaceuticals Inc | Compositions and methods for inhibiting expression of transthyretin | 2012-05-01 |
| US20130281510 | Ando et al. | Kumamoto University, Alnylam Pharmaceuticals Inc | siRNA Therapy for Transthyretin (TTR) Related Ocular Amyloidosis | 2013-10-24 |
| US9101643 | Dinah Wen-Yee Sah | Alnylam Pharmaceuticals Inc | Lipid formulated compositions and methods for inhibiting expression of transthyretin (TTR) | 2015-08-11 |
| US9200284 | Bob D. Brown | Dicerna Pharmaceuticals Inc | Methods and compositions for the specific inhibition of KRAS by asymmetric double-stranded RNA | 2015-12-01 |
| US9234196 | Wen-Yee Sah et al. | Alnylam Pharmaceuticals Inc | Compositions and methods for inhibiting expression of transthyretin | 2016-01-12 |
| US9422556 | Chatterton et al. | Arrowhead Pharmaceuticals Corp | RNAi-related inhibition of TNF-alpha signaling pathway for treatment of ocular angiogenesis | 2016-08-23 |
| Delivery Methods | ||||
| US7404969 | Chen et al. | Sirna Therapeutics Inc | Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules | 2008-07-29 |
| US20100285112 | Novobrantseva et al. | Arbutus Biopharma Corp | Methods of delivering oligonucleotides to immune cells | 2010-11-11 |
| US8106022 | Manoharan et al. | Alnylam Pharmaceuticals Inc | Carbohydrate conjugates as delivery agents for oligonucleotides | 2012-01-31 |
| US8642076 | Manoharan et al. | Arbutus Biopharma Corp | Lipid containing formulations | 2014-02-04 |
| US9139554 | Hope et al. | University of British Columbia, Arbutus Biopharma Corp | Amino lipids and methods for the delivery of nucleic acids | 2015-09-22 |
| US9453222 | Manoharan et al. | Alnylam Pharmaceuticals Inc | Ligand-conjugated monomers | 2016-09-27 |
| US9526796 | David B Rozema, Darren H Wakefield | Arrowhead Pharmaceuticals Corp | Peptide-based in vivo siRNA delivery system | 2016-12-27 |
[1]Footnote 6, Roche Molecular Systems, Inc. v. Cepheid (Fed. Cir. 2018).
–David Puleo and Anthony D. Sabatelli, PhD, JD
This article is for informational purposes, is not intended to constitute legal advice, and may be considered advertising under applicable state laws. The opinions expressed in this article are those of the author only and are not necessarily shared by Dilworth IP, its other attorneys, agents, or staff, or its clients.

