The I-O Movement: Priming the Immune System to Fight Cancer – Part I: Chimeric Antigen Receptor T (CAR-T) Cell Technology
Mar 27th, 2018 by David Puleo | News | Recent News & Articles |
This is the first article in a Series focused on current trends in immuno-oncology (I-O) and immunotherapy. Since the latter are becoming household terminology, the goal of this Series is to highlight the value of intellectual property for new immunotherapies in this burgeoning field.
There has been a lot of recent buzz about chimeric antigen receptor (CAR) T cell technology. Novartis and Gilead have FDA-approved CAR-T therapies offering complete patient remission from certain cancers. A flood of new CAR-based technologies is likely to hit the market. As with any novel therapy, patent protection is essential. The large number of patent filings suggest that intellectual property protection is an important part of the research efforts in this field. The bolded patent documents cited in this article are further summarized in the table at the end of this installment.
CAR-T Background
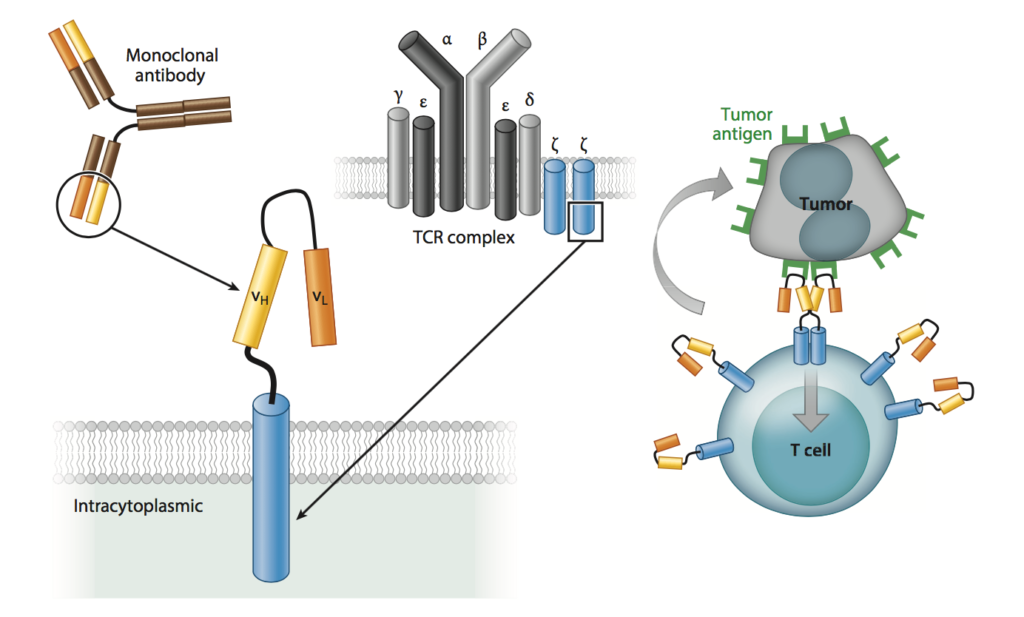
CAR-T was developed in the 1990s, but we are just starting to hear about it now. So, what is it exactly? Essentially, immune cells called T cells are removed from a patient’s body, re-engineered to target a specific cancer, then put back into the patient to fight that specific cancer. When the CAR binds the tumor-associated antigen to which it is directed, the T cell is activated, eliciting an immune response (Figure 1).
Figure 1: CAR structure and function. [Source: Annu. Rev. Med. 67, 165–83 (2016).]
To get more in depth, CARs are essentially modified T-cell receptors (TCR) consisting of an extracellular (outside of the cell) antigen binding domain, most commonly an antibody fragment known as an scFv, that binds to an antigen on a cancer cell. The scFv is fused via a transmembrane domain to an intracellular (inside of the cell) domain of a TCR.
Firstly, T cells are obtained by a simple blood draw from the patient. After the T cells are separated from the blood (a process called leukapheresis) and further cultured, DNA encoding the engineered CAR is inserted into and then expressed by the patient’s T cells. Patent applications US20150344844 and US20170218337 disclose methods of preparing and manufacturing CAR-T cells. Typically, chemotherapy is used to deplete a patient’s existing T cells in preparation for infusion of the re-engineered CAR-T cells back into the patient. This preparatory method is detailed in US20170368101.
The first-generation CARs included one intracellular portion of a TCR. However, this portion alone did not generate an efficient immune response. Further iterations of CAR-T therapies added more co-stimulatory TCR fragments, which allowed for improved activation and proliferation. There are also bispecific CARs which recognize and require binding of two separate antigens, thus increasing the specificity of T cell signaling. Patent US9447194 and patent applications WO2016130598 and US20160303230 detail the uses of bispecific CARs.
The CAR-T Patent Landscape
The following sections will review CAR-T therapies targeting different antigens. This is by no means a complete list of all therapies; rather, the following sections are meant to give the reader a brief snapshot of the CAR-T patent landscape:
Anti-CD19 CAR-T
Although a number of CAR-T therapies are being used to treat CD19-positive liquid cancers, such as lymphomas and leukemias, the CAR-T field was initially riddled with doubt. Juno Therapeutics’s lead therapy, JCAR015, was used to treat adult lymphoblastic leukemia. However, during clinical trials, five patients died due to cytokine release syndrome (CRS; also known as a “cytokine storm”). As the name suggests, there is a rapid release of (pro-inflammatory) cytokines, which causes fever-like symptoms and massive brain swelling. As a result, JCAR015 development has halted. Although Juno does not currently have an FDA-approved CAR-T therapy, recent data have shown that their anti-CD19 therapy targeting Non-Hodgkin Lymphoma, JCAR017, is promising. Patent application US20160152723 discloses Juno’s anti-CD19 CAR-T. Of note, Juno was recently acquired by Celgene for $9B.
The first FDA-approved anti-CD19 CAR-T therapy, KymriahTM (tisagenlecleucel/CTL019), is being used to treat relapsed or refractory (r/r) B-cell acute lymphoblastic leukemia (ALL). KymriahTM was developed by Novartis in collaboration with the University of Pennsylvania (UPenn) and Children’s Hospital of Pennsylvania, the early work of which was described in detail [Sci. Transl. Med. 3, 95ra73 (2011)]. Patent applications US20140271635 and US20150283178 describe Novartis’s anti-CD19 CARs.
Similarly, Kite Pharma’s anti-CD19 YescartaTM (axicabtagene ciloleucel) was recently approved for the treatment of r/r large B-cell lymphoma, including diffuse large B-cell lymphoma (DLBCL) and several other subsets. Patent application US20150344844 describes this work, which was performed by Kite in collaboration with the National Cancer Institute. Of note, Kite was recently acquired by Gilead Sciences to the tune of $11.9B, putting Gilead in prime position to be at the top of the CAR-T I-O arena. However, Novartis is currently seeking FDA-approval for KymriahTM in adult r/r DLBCL, for which it has positive clinical data. If approved for DLBCL, KymriahTM will be in direct competition with YescartaTM.
Anti-BCMA CAR-T
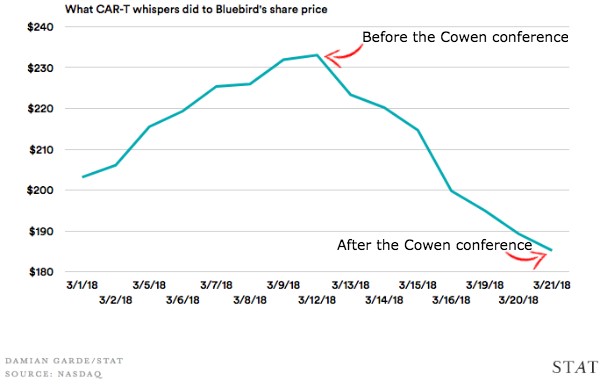
Patent US9765342 (U.S. Department of Health and Human Services) and patent applications US20160046724 (Novartis/UPenn), US20160297884 (Pfizer), US20170183418 (Cellectis), US20170226216 (bluebird bio), and US20170283504 (Kite Pharma) describe a CAR-T therapy that targets B-cell maturation antigen (BCMA). The bispecific CAR disclosed in WO2016130598 also binds BCMA. The BCMA protein is prevalent on multiple myeloma (MM) cells. In particular, MM patients have shown excellent response rates with bluebird/Celgene’s bb2121 in Phase I trials and is moving to Phase II. Recently, bb2121 was granted Breakthrough Therapy Designation by the FDA. However, that success was dampened by news released earlier this month at the 2018 Cowen Healthcare Conference indicating that three patients relapsed on bb2121. bluebird’s stock price was immediately effected (Figure 2). In collaboration with Celgene, bluebird is also testing its second generation BCMA therapy, bb21217. Novartis’s CART-BCMA has shown promising results in a small Phase I trial. Kite Pharma recently submitted an Investigational New Drug application in order to initiate a Phase I trial for its anti-BCMA therapy, KITE-585.
Figure 2: bluebird share price over the month of March [Source: STAT]
Regulatable CAR-T
CAR-T cells can undergo uncontrolled proliferation. As such, new CARs are being generated that contain inducible regulatory elements to prevent such unwanted effects. Specifically, induction can be used to initiate a CAR-T cell immune response. Patent applications US20140286987 and US20160046700 describe such inventions. Furthermore, there are some CARs that contain inducible elimination or suicide genes to allow for selective depletion of CAR-T cells. Patent US9447194 and applications US20130071414 and WO2016130598 describe CARs with such inducible genes.
CAR-T: Moving Forward
While a number of the earlier CARs are being used to target liquid cancers, some are currently in development to treat solid tumors. This type of treatment is inherently challenging because the CAR-T cells would need to infiltrate both solid tumor sites as well as the tumors themselves. For example, the mutant variant III of EGFR, EGFRvIII, causes glioblastoma; CAR-T cells have been shown to limit tumor growth [Sci. Transl. Med. 7, 275ra22 (2015)]. Multiple patents, including US9394368 and US9624306, detail CAR-T therapies targeting EGFRvIII. Belliucm’s patent application US20140023647 describes CAR-T therapy for the treatment of solid tumors, specifically prostate cancer. Furthermore, chondroitin sulfate proteoglycan-4 (CSPG4) has been identified as a protein antigen that is highly expressed in glioblastoma. Thus, CAR-T therapies are being developed against CSPG4 [Sci. Transl. Med. 10, eaao2731 (2018)]. Patent US20160376375 discloses this technology.
Pricing is another separate, yet important, issue. KymriahTM and YescartaTM have price tags of $475,000 and $373,000, respectively. However, this is just the cost of the therapy itself. The total cost of care is estimated to be around $1.5M per patient. Specifically, Express Scripts has been vocal about the need for new business and payment models in the gene therapy space. It will be interesting to determine what developments will happen in this area and, furthermore, revisit the idea of patent protection when the patent lifetime of these therapies runs out. This is an extremely exciting time to be in the I-O space.
Table 1: Patent and Patent Applications
| Patent Number | Inventor | Assignee | Title | Issue or Publication Date |
| US20130071414 | Dotti et al. | Baylor College of Medicine | Engineered cd19-specific t lymphocytes that coexpress il-15 and an inducible caspase-9 based suicide gene for the treatment of b-cell malignancies | March 21, 2013 |
| US20140023647 | Slawin et al. | Baylor College of Medicine, Bellicum Pharmaceuticals, Inc. | Method for treating solid tumors | Jan 23, 2014 |
| US20140271635 | Brogdon et al. | The Trustees of The University of Pennsylvania, Novartis Ag | Treatment of cancer using humanized anti-cd19 chimeric antigen receptor | Sept 18, 2014 |
| US20140286987 | David Spencer, Aaron Edward Foster | Bellicum Pharmaceuticals, Inc. | Methods for controlling t cell proliferation | Sep 25, 2014 |
| US20150283178 | June et al. | Novartis AG, University of Pennsylvania | Treatment of cancer using anti-cd19 chimeric antigen receptor | Oct 8, 2015 |
| US20150344844 | Better et al. | US Department of Health and Human Services (HHS), Kite Pharma Inc | Methods for producing autologous t cells useful to treat b cell malignancies and other cancers and compositions thereof | Dec 3, 2015 |
| US20160046724 | Brogdon et al. | Novartis AG, University of Pennsylvania | Treatment of cancer using humanized anti-bcma chimeric antigen receptor | Feb 18, 2016 |
| US20160046700 | Foster et al. | Bellicum Pharmaceuticals, Inc. | Methods for activating t cells using an inducible chimeric polypeptide | Feb 18, 2016 |
| US20160152723 | Chen et al. | Juno Therapeutics, Inc. | Antibodies and chimeric antigen receptors specific for cd19 | Jun 2, 2016 |
| US9394368 | Brogdon et al. | Novartis AG, University of Pennsylvania, University of Pittsburgh | Treatment of cancer using humanized anti-EGFRvIII chimeric antigen receptor | Jul 19, 2016 |
| WO2016130598 | Lung-Ji Chang | University of Florida Research Foundation, Inc. | Bi-specific chimeric antigen receptor and uses thereof | Aug 18, 2016 |
| US9447194 | Michael Jensen | Seattle Children’s Hospital | Bispecific chimeric antigen receptors and encoding polynucleotides thereof | Sep 20, 2016 |
| US20160297884 | Kuo et al. | Pfizer, Inc. | Chimeric antigen receptors targeting b-cell maturation antigen | Oct 13, 2016 |
| US20160303230 | Ahmed et al. | Baylor College of Medicine | Chimeric antigen receptor for bispecific activation and targeting of t lymphocytes | Oct 20, 2016 |
| US20160376375 | Gianpietro Dotti, Soldano Ferrone | Baylor College of Medicine | CSGP4 – Specific Chimeric Antigen Receptor for Cancer | Dec 29, 2016 |
| US9624306 | Richard A. Morgan, Steven A. Rosenberg | US Department of Health and Human Services (HHS) | Anti-epidermal growth factor receptor variant III chimeric antigen receptors and use of same for the treatment of cancer | Apr 18, 2017 |
| US20170183418 | Roman Galletto | Cellectis SA | Bcma (cd269) specific chimeric antigen receptors for cancer immunotherapy | Jun 29, 2017 |
| US20170218337 | Kevin Friedman | Bluebird Bio Inc. | Improved t cell compositions | Aug 3, 2017 |
| US20170226216 | Richard Morgan, Kevin Friedman | Bluebird Bio, Inc. | Bcma chimeric antigen receptors | Aug 10, 2017 |
| US9765342 | James Noble Kochenderfer | US Department of Health and Human Services (HHS) | Chimeric antigen receptors targeting B-cell maturation antigen | Sep 19, 2017 |
| US20170283504 | Jed Wiltzius, Ruben Alvarez Rodriguez | Kite Pharma, Inc. | Bcma binding molecules and methods of use thereof | Oct 5, 2017 |
| US20170368101 | Bot et al. | US Department of Health and Human Services (HHS) Kite Pharma Inc | Methods of conditioning patients for t cell therapy | Dec 28, 2017 |
–David Puleo and Anthony D. Sabatelli, PhD, JD
This article is for informational purposes, is not intended to constitute legal advice, and may be considered advertising under applicable state laws. The opinions expressed in this article are those of the author only and are not necessarily shared by Dilworth IP, its other attorneys, agents, or staff, or its clients.