Nature’s Indelible Marker
Sep 11th, 2014 by Michael Dilworth | News | Recent News & Articles |
Naturally abundant, stable isotopes have already protected the identity and origin of $1.5 billion in pharmaceutical materials against intellectual property infringers
Editor’s note: This article first appeared in the digital and print editions of Pharmaceutical Manufacturing Magazine in September 2014. The author, Dr. Anthony Sabatelli, retains the rights to the piece and has been given permission for this article to be reprinted here. Molecular Isotope Technologies LLC is a client of Dilworth IP and Dr. Sabatelli has assisted MIT LLC in pursuing patent protection for the technology discussed in this article.
The pharmaceutical industry is an important segment of the world economy. In 2013, worldwide sales of prescription drugs were over $830 billion. If one adds in over-the-counter medicines and other health-related products such as vitamins and nutritional supplements, that market approaches nearly $1 trillion dollars annually. With such huge markets at stake, there is the continuous threat of unauthorized sales of competing counterfeit and patent-infringing pharmaceutical products.
In an effort to find a simple, accurate way to unequivocally identify and secure the origins of drugs and other compounds, a Niantic, Connecticut-area scientist developed an alternative, finding a way to use the naturally occurring molecular fingerprint of drug products, active pharmaceutical ingredients and their synthetic pathways to help biotech and pharmaceutical companies enforce their hard-won patent rights against counterfeiters and other bad actors looking to profit by stealing intellectual property.
According to Dr. John Jasper, chief scientific officer for Nature’s Fingerprint, a division of Molecular Isotope Technologies LLC (MIT LLC), this fingerprinting technology has real, practical, application potential. “What we are doing is measuring chemical tracers, also known as stable isotopes, to determine the origins of drug products and pathways to help pharmaceutical companies and law enforcement authorities combat Intellectual property infringement,” says Jasper. “The distribution of natural-abundance stable isotopes in a drug product is analogous to the highly specific pattern of a human fingerprint. It is this ‘fingerprint’ of nature that can be used to highly specifically identify the source or process for a given drug product.”
The History of Isotopes
Jasper explains that large-scale scientific research involving both radioactive and non-radioactive (i.e., stable) isotopes goes back to the time of the Manhattan Project, which produced the first atomic bombs during World War II. Isotopes — whether stable or radioactive — are forms of the same chemical element having different atomic masses. For example, uranium has an isotopic form with a mass of 235 and also an isotopic form with a mass of 238. By 1942, isotopes had only been known for about 30 years. Most of the research involving isotopes had been theoretical, relating to determining atomic structures and studying the then-mysterious properties of radioactivity. The Manhattan Project changed all that. The dire urgency of the war effort led to the development of sophisticated techniques for separating and identifying isotopes. One of these techniques, Isotope Ratio Mass Spectrometry (or IRMS for short), which is used to measure the relative abundance of isotopes in a sample, is now an important methodology in the field of those studying and using isotopes.
Highly Specific Fingerprint
In 1999, Jasper began developing the technology around the premise that naturally abundant, stable isotopes might be used to identify individual batches of pharmaceutical materials. According to Jasper, “we had shown that every batch of pharmaceutical products had its own highly specific ‘isotopic fingerprint.’ We subsequently realized the potential applications of our technology to provide evidentiary support for patent infringement and enforcement efforts had just opened up immensely.”
Two U.S. Patents
The technology is sufficiently unique that it was awarded two U.S. patents, the most recent being issued in 2013. The latest patent relates to methods and systems to correlate a product such as a pharmaceutical to the synthetic process by which it was made. In other words, the method is a means of finding the “smoking gun” of the patent infringer. Even the FBI has recognized the value of this unique technology, calling upon Jasper for advice during the anthrax scare post 9/11.
Adding Value
It is well recognized that pharmaceutical research is a high-risk and expensive undertaking. It takes many years and huge investments to bring a new drug product to the marketplace. This is not surprising considering that a single human Phase III clinical trial can easily top $50 million dollars to run. Phase III is the last stage of clinical testing before a pharmaceutical developer submits an application for drug approval to the FDA. The FDA typically requires two such “well-controlled” studies for drug approval. According to the Tufts Center for the Study of Drug Development, which has been tracking the cost of prescription drug development for over 30 years, the cost of developing a single new drug today is estimated to cost as much as $1.2 billion. On top of this immense cost, the average time for bringing a new drug to market from its original inception in the lab is now over 10 years. Finally, the success rate for prescription drug development is quite low — by some estimates, only about one in 20 drugs make it all the way from initial Phase I human clinical trials to the marketplace. Going back even further to inception at the lab bench, the success rate might be just one in several thousand.
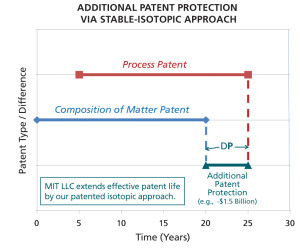
Plot showing the period of additional patent protection potentially afforded by the use of MIT LLC’s stable-isotopic approach to process patent protection. Click image to enlarge.
The main way to secure protection for new pharmaceutical products is through sound patent protection. However, the cost of patent protection is high and time consuming. The “gold standard” in patent protection for a new, small organic molecule drug product is a composition of matter patent. Such patents specifically describe or “claim” the drug compound, and preferably a broader genus of chemical structures surrounding that compound. Furthermore, the cost for filing, obtaining and maintaining a single patent across a broad range of countries can cost tens of thousands of dollars, and in many instances can easily top a half a million dollars.
However, it is not always possible to obtain a “composition of matter” patent. In other instances, because of long drug development timelines and stringent regulatory requirements and review, a significant amount of the patent term (typically 20 years) of the composition of matter patent has already ticked away by the time the product has been approved for marketing. Even though the U.S. Patent Office, in conjunction with the FDA, provides for up to five years of patent term restoration, the restored patent term can still be relatively short. In many cases, composition of matter patents may have expired or may not otherwise be available for reasons having nothing to do with the soundness, value or utility of the underlying drug product.
Whether or not there is a composition of matter patent, pharmaceutical companies often supplement their patent portfolios with patents covering manufacturing processes and improvements (i.e., process patents). These patents are sometimes referred to as secondary patents. These patents can relate to more efficient processes or products with improved purity profiles or properties. However, these secondary patents can be more difficult to monitor and subsequently enforce. The reason for this is that the evidence necessary to substantiate a claim of infringement is not as easy to obtain or demonstrate as in the case of a composition of matter patent.
Counterfeiting of pharma products and infringement of patent processes are multi-billion-dollar problems for the pharmaceutical industry. Here is where the technology developed by Dr. Jasper can potentially be successful. Figure 1 graphically illustrates the period of additional patent protection potentially afforded by the use of MIT LLC’s stable-isotopic approach to process patent protection.
Identifying Drug Sources
How can isotopic fingerprinting technology differentiate the source of a drug and/or the process by which it is made? “All chemical compounds have highly specific ratios of natural stable isotopes,” explains Jasper. Thus every batch of a pharmaceutical material has its own highly specific isotopic fingerprint. The method can be used to identify, track and classify batches of products. The technology can identify not only the type of chemical processes used in making a drug, but also the identifying information of the specific manufacturing site as well.”
Very Sensitive
This fingerprinting technology provides a means to investigate and determine facts, facts that can prove provenance and process. Many times it is difficult to monitor and enforce process patents because of the difficulty of finding persuasive evidence of infringement. The reason isotopic evidence can be so persuasive is because it is so sensitive. The technology can measure very small differences in natural isotopic ratios between samples. Because of this sensitivity the method can, with a very high degree of certainty, determine whether two or more samples or processes are the same or different. According to Jasper, “we can perform very highly precise analyses on samples that are only a fraction of a milligram.” (To put this in perspective, a typical grain of salt weighs less than a milligram.)
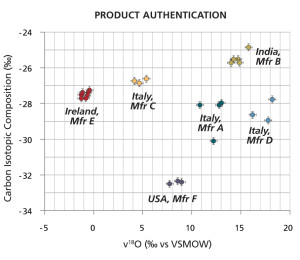
Determination of the manufacturing source for naproxen samples. MIT LLC’s fingerprinting technology was used, in a blind study commissioned by the FDA, to determine the manufacturing source for 26 separate batches of naproxen obtained from various sites around the world. By determining the naturally occurring isotope ratios for the carbon and oxygen isotopes in the samples, as shown in the graph to the left, Dr. Jasper and his team successfully determined that the 26 batches had come from six different manufacturing sources. Click image to enlarge.
In 2005, in a blind study commissioned by the FDA, MIT LLC characterized 26 batches of the pain reliever, naproxen, to determine the product fingerprint or source characteristics for the batches. The FDA wanted to assess the robustness of the fingerprinting method for determining the manufacturing sites for pharmaceutical products. If the method could provide this source information, it would be an important investigative and forensic tool for drug enforcement authorities trying to determine the provenance of a product. For this study, Jasper and his team determined the ratios of the naturally occurring carbon and oxygen isotopes for each of the naproxen batches. It turns out Jasper and his team had correctly determined that the batches had come from six different manufacturing sites from around the world (See Figure 2).
Potential Applications
To date, Jasper’s technology has been employed in three lawsuits, with two lawsuits being decided in favor of plaintiffs claiming patent infringement against counterfeiters. Further, his technology also helped another defendant ward off an unfounded patent infringement claim. MIT LLC conducted a study for this defendant comparing the naturally occurring carbon-isotope ratios for the defendant’s products versus the ratios for a product made by the plaintiff’s patented process. The data unequivocally showed that the defendant’s product could not have been made by the patented process as alleged by the plaintiff. Based on the data, the defendant was found not guilty of patent infringement and they were free to bring their product into a major, new international market (See Figure 3).
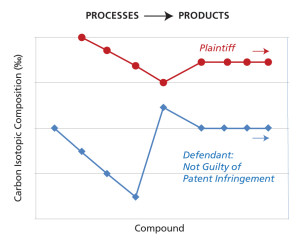
Non-Infringement of a patented process. MIT LLC provided data used in the successful defense against a claim of patent infringement. The MIT LLC team conducted a study showing that the naturally occurring carbon isotope ratios in the defendant’s products were distinctly different from those of the plaintiff patent holder’s process. This difference is dramatically shown from the graph to the left. Click image to enlarge.
These results highlight the potential impact of this fingerprinting technology, particularly to adding value to a company’s patent estate. A conservative estimate puts the value of the products protected by the technology at more than $1.5 billion in total sales over the time the settlements were in effect.
More Innovation Coming
What’s next for Jasper’s innovative identity tool? Jasper says MIT LLC continues to expand its business development outreach while working to develop new isotope analysis tools. For example, Nature’s Fingerprint has developed new technology to evaluate naturally occurring isotopes in high molecular weight molecules and complex biological systems. Recent studies, says Jasper, have demonstrated the successful application of the technology to biologic compounds in the 8,000 to 15,000 dalton molecular-weight range (Jasper, et.al., J. Pharm. Sci. 2014, in press). Such high molecular-weight compounds can be more than two orders of magnitude larger than the typical low-molecular weight drug compounds. For comparison, naproxen has a molecular weight of 230. Jasper pointed out that in principle, there is really no upper limit to the size molecules or systems to which his technique can be applied, but rather it is ancillary issues such as sample preparation and purification that may present some challenge.
Additionally, the company has developed technology for continuously monitoring the progress of chemical and biological reaction processes. This technology is based on the partitioning of natural-abundance stable isotopes between the reactants and products of a chemical or biological process through the course of a reaction. The technique can have a wide range of applications from monitoring the progress of a chemical reaction in a pilot plant to the large-scale production of commodity chemicals. In theory, the isotopic composition of a reaction product can be plotted as a function of reaction yield. For example, there is good reason to believe that the stable-isotopic composition of reaction products should be proportional to the instantaneous yield of the reaction. That is, the isotopic composition (δ) should increase as the reaction yield for the process approaches completion (i.e., 1.0). The symbol, ‰, designates per mil (a/k/a parts per thousand). Jasper says he is positive about this development because it applies the isotope technology to solving real-time monitoring problems for process chemistry and other manufacturing processes (See Figure 4).
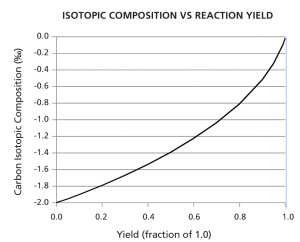
This graph illustrates the principle that the stable-isotopic composition of reactants is related to the yield of a reaction. The isotopic composition should increase as the reaction yield for the process approaches completion (i.e., 1.0). The symbol, ‰, designates permil or what is also referred to as parts per thousand. Click image to enlarge.
Things have come a long way from the early 1900s when researchers first postulated the existence of different isotopic forms of the chemical elements. Nature’s Fingerprint has found a unique application of this isotope technology. It is a company to watch, particularly as it continues to add value to and protect the pharma industry’s intellectual property.
This article is for informational purposes, is not intended to constitute legal advice, and may be considered advertising under applicable state laws. The opinions expressed in this article are those of the author only and are not necessarily shared by Dilworth IP, its other attorneys, agents, or staff, or its clients.